Ozone's Dilemma with HNO3-doped Polar Stratospheric Clouds
Synopsis of the NASA Ames aerosol group's contribution
Scheme
Several hypotheses were advanced in the 1980s that tried to explain the cause of the ozone hole that had been discovered over Antarctica. This discovery was important, because the ozone layer protects human, animal and plant life from the powerful (UV) rays of the sun. When the ozone layer weakens, more ultraviolet-B rays reach the Earth's surface, making humans more prone to skin cancer, cataracts and other diseases. Understanding ozone depletion thus became an important task.
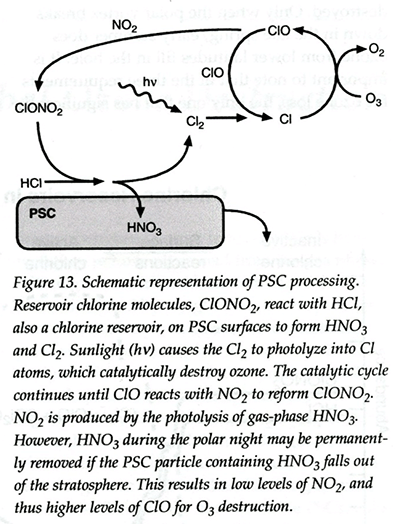
One explanation given of the cause of ozone depletion invoked catalysis of nitrate transformations on or in polar stratospheric cloud (PSC) particles that potentially alters the standard relationship between benign chlorine reservoirs, HCl and ClONO2, and ozone-reactive ClOx radicals in the polar regions. Figure 1 (Fig. 13 in Middleton and Tolbert, 2000) depicts the phenomenon: Ozone-friendly reservoir chlorine molecules, ClONO2 react with likewise ozone-friendly HCl on PSC surfaces to form HNO3 and Cl2. Ultraviolet photons (hν) from sunlight cause the Cl2 to form Cl-atoms by photolysis that catalytically destroy ozone. The catalytic cycle continues until ClO reacts with NO2, produced by photolysis of gas-phase HNO3, to again form ClONO2. However, HNO3 attached to PSC particles may be permanently removed from the atmosphere when the PSC particles containing HNO3 settle out of the stratosphere during polar nights. Such denitrification results in lower levels of NO2, thus higher levels of ClO for O3-destruction. Such denitrification would thus prolong the duration of the ozone-hole (Wofsy et al., 1990).
An experimental proof of the correctness of this scheme was to show that PSC particles indeed contain nitrate. This became our challenge in AAOE. If positive, this finding would lend credibility to the theory that the surfaces involved in heterogeneous chemical schemes are formed from nitric acid that had condensed on PSC particles. Further, we considered the possibility that HNO3 removal by settling out of the atmosphere of PSC particles could denitrify the stratosphere, thus removing HNO3 which otherwise would provide a photolytic source of NO2 to allow ClO to be re-converted to the inert reservoir species ClONO2.
Method
During AAOE we sampled stratospheric cloud particles between 57° and 72° southern latitude at 18 km altitude by inertial impaction on Ames wire impactors (Farlow et al., 1979). The impactors were mounted on the left wingtip of the ER-2 research aircraft. Each of six impactors, consisting of four 500 μm diameter gold wires strung across six 2.5 cm diameter support rings, were sequentially exposed at 6 different times of each flight. The wires were pretreated to permit observation of chemical and morphological particle characteristics.
One of four wires was coated with NitronT to induce a chemical reaction that is specific to nitrate (Mamane and Pueschel, 1980). Three other wires were coated with barium chloride and silver nitrate, respectively, to induce reactions that specify sulfates and chloride (Mamane and DePena, 1978; Preining et al., 1976; Yue and Podzimek, 1980). After collection, the diagnostic reaction spots were analyzed and counted in a scanning electron microscope. Their concentration was assessed in relation to the concentration of the total aerosol determined by counting and sizing the deposits on carbon-coated wires. Additional X-ray energy dispersive analysis of single particles on the wire constituted a second method of verifying the presence of sulfur and chlorine in the particles, in addition to micro-chemical spot tests.
Results
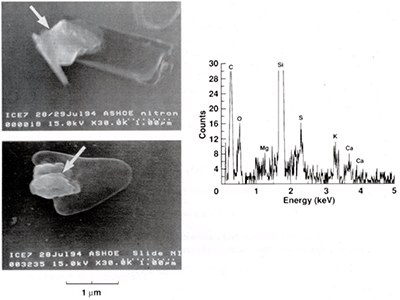
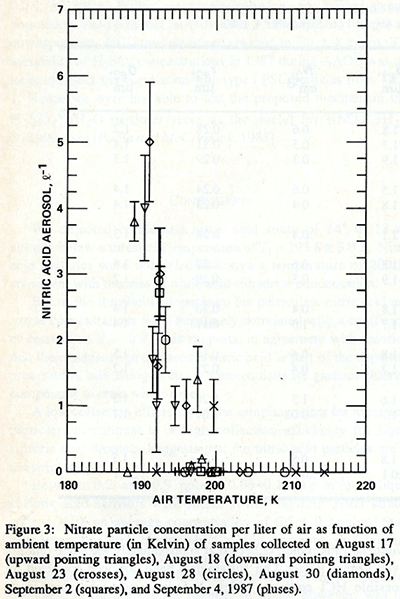
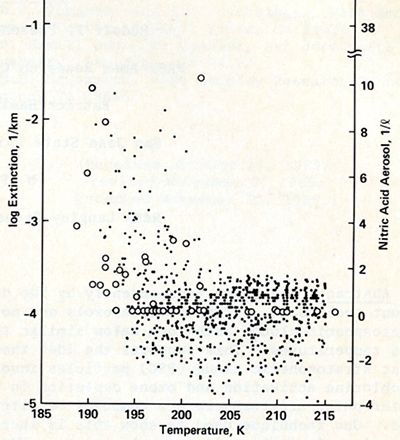
Figure 2 is a typical example of our findings. The picture shows scanning electron microscope images of columnar and triangular ice particles. The brighter parts at some edges of these particles are NitronT-nitrate reaction spots that prove the attachment to the particles of nitrate. To the right of the PSC-cloud particle images is shown their X-ray energy dispersion spectrum. The sulfur emission might be of significance, because sulfuric acid particles from volcanic eruptions could produce similar redistribution of halogen compounds at lower than polar latitudes (Verdecchia et al., 1992), similar to the action of PSC particles near the poles.
A summary of results from 6 flights during AAOE are shown in Fig.3A (Pueschel et al., 1989). A RMS-square fit through the data resulted in a threshold temperature of nitric acid formation of Tt=193.6 ± 3.0 K (intercept with the abscissa). This finding is proof that PSCs in the cold Antarctic stratosphere contain nitric acid n-hydrate (type 1 polar stratospheric cloud particles, which according to Toon et al., 1986, first condense at about 193 degrees Kelvin, while pure water particles would condense only if the temperature reached 187 Kelvin). Nitric acid particles were never detected above 200 K (Pueschel et al., 1989).
However, our technique of stratospheric aerosol collection is labor-intensive and expensive, due to the fact that it requires a special aircraft as an in-situ sampling platform. Therefore, we have combined our finding with that of the Stratospheric Aerosol Measurement (SAM II) satellite (McCormick et al., 1987 and 1989).
Figure 4 shows the nitric acid aerosol concentration per liter (circles, right hand coordinate) and the one micrometer light extinction per kilometer (dots, left hand coordinate). Averaging all data points above zero nitric acid particle concentration and above 3E-4 light extinction as function of temperature, and fitting RMS curves to the data, resulted in threshold temperatures for nitric acid aerosol of Tt,1 = 194 ± 4 K and of enhanced light extinction of Tt,2=196 ± 3 K. Those threshold temperatures are equivalent within the RMS- uncertainty. Thus, enhanced light extinction as detected by SAM II implicitly is partly due to nitrate-containing PSC particles.
Acknowledgments
In late 1970, a young Israeli scientist from the Technion in Haifa walked into my office and introduced himself as Yaakov Mamane. He had been awarded a NOAA postdoctoral fellowship that he wanted to apply to aerosol research. His expertise in nitrate particle detection, then very timely in terms of air pollution research needs, raised my immediate attention. He set out to perfect the technique during the time of our collaboration that, in due time, I called to AAOE management's attention.
When asked to participate in AAOE in 1987, I played with the idea of bringing back Dr. Mamane to help us detect nitrate aerosol in PSC particles. But the Ames Aerosol Group already had been collaborating extensively for many years with Jindra Goodman, Professor of Meteorology at San Jose State University. Of particular interest to AAOE was her expertise in ice crystal detection. Thus we chose Prof. Goodman to apply her proficiency to nitric acid particle detection which she agreed to and exercised exceptionally well.
Back in the laboratory we enjoyed the collaboration with Sunita Verma of Norcal Corporation. Her expertise and help in electron microscopy likewise was suitable to our research and is greatly appreciated and acknowledged. Dr. Kenneth Snetsinger of NASA Ames oversaw and managed cloud particle counting and analysis.
Last but not least, Professor Margarita Prendez of the University of Santiago acted as liaison between the Ames Aerosol Group and Chilean authorities that showed interest in the explanation of cause end effects of the ozone hole.
Conclusion
Detection of nitrate in PSC particles was but a small piece out of many that made up the "ozone hole" puzzle which, in the 1980s, posed a huge environmental problem. Nevertheless, we were able to contribute to its solution which, in turn, led to an international agreement, known as the Montreal Protocol, that demanded a world-wide phasing out of the emission of ozone depleting chemicals, including chlorofluorocarbons (CFCs) from refrigerants and aerosol spray propellants. CNN News announced lately, that "the hole in the Earth's ozone layer is expected to fully heal within 50 more years," referring to findings of the UN's International Panel of Climate Change (IPCC). Thus, AAOE initiated a "healing process" that is expected to eradicate the Ozone hole sometime in the future. Having participated in this endeavor occasioned the most rewarding experience of my career.
References
- Farlow, N.H. et al., Latitudinal variations of atmospheric aerosols, Journal of Geophysical Research, 84, 733-743, 1979
- Mamane, Y. and R.F. Pueschel, A method for the detection of individual nitrate particles, Atmospheric Environment, 14, 629-639, 1980
- Mamane, Y. and R.G. dePena, A quantitative method for the detection individual submicron size sulfate particles, Atmospheric Environment, 12, 69-82, 1978
- McCormick, M.P. et al., Characteristics of polar stratospheric clouds as observed by SAM II, SAGE and lidar, Journal of the Meteorological Society of Japan, 63, 267-276, 1985
- Middleton, A.M. and M.A. Tolbert, Stratospheric Ozone Depletion, University Science Books, Sausalito, CA, ISBN 1-891389-10-6, 2000
- Preining, O. et al., The magnification factor for sodium chloride Liesegang circles established with an aerosol centrifuge, Journal of Aerosol Science, 7, 351-4358, 1976
- Pueschel, R.F., Stratospheric Aerosol Formation, Properties, Effects, Journal of Aerosol Science, 27, 383-402, 1996
- Pueschel, R.F. et al., Condensed Nitrate, Sulfate and Chloride in Antarctic Stratospheric Aerosols, Journal of Geophysical Research, 94, 11271-11384, 1989
- Pueschel, R.F. et al., Nitric Acid in Polar Stratospheric Clouds, Geophysical Research Letters, 17, 429-432, 1990
- Pueschel, R.F. et al., A Case of Type I Polar Stratospheric Cloud Formation by Heterogeneous Nucleation, Journal of Geophysical Research, 97, 8105-8114, 1992
- Toon, O.B. et al., Condensation of HNO3 and HCl in the winter polar stratosphere, Geophysical Research Letters, 13, 1284-1287, 1986
- Verdecchia, M. et al., Radiative perturbation due to the eruption of El Chichon - effects on ozone, Journal of Atmospheric and Solar-Terrestrial Physics, 54, 1081-1084, 1992
- Wofsy, S. et al., Condensation of HNO3 on falling ice crystals: mechanism for denitrification of the polar stratosphere, Geophysical Research Letters, 17, 449-452, 1990