Scientific Assessment of Ozone Depletion: 1998
Frequently Asked Questions About Ozone

Ozone is very rare in our atmosphere, averaging about three molecules of ozone for every 10 million air molecules. In spite of this small amount, ozone plays vital roles in the atmosphere. This appendix to the Executive Summary of the Scientific Assessment of Ozone Depletion: 1998 provides answers to some of the questions that are most frequently asked about ozone and the changes that have been occurring in recent years. These questions and answers are based on the information presented in this 1998 report, which was prepared by 304 scientists from 35 countries worldwide. Therefore, the information presented here represents the knowledge of a large group of experts from the international scientific community.
Ozone is mainly found in two regions of the Earth's atmosphere. Most ozone (about 90%) resides in a layer between approximately 10 and 50 kilometers (about 6 to 30 miles) above the Earth's surface, in the region of the atmosphere called the stratosphere. This stratospheric ozone is commonly known as the "ozone layer." The remaining ozone is in the lower region of the atmosphere, the troposphere, which extends from the Earth's surface up to about 10 kilometers. The figure below shows this distribution of ozone in the atmosphere.
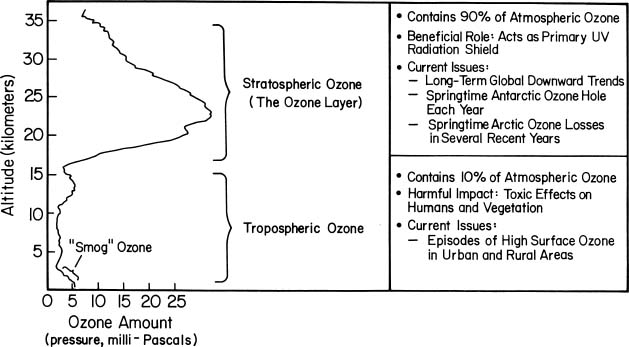
The ozone molecules in these two regions are chemically identical, because they all consist of three oxygen atoms and have the chemical formula O3. However, they have very different effects on humans and other living beings. Stratospheric ozone plays a beneficial role by absorbing most of the biologically damaging ultraviolet sunlight (called UV-B), allowing only a small amount to reach the Earth's surface. The absorption of ultraviolet radiation by ozone creates a source of heat, which actually forms the stratosphere itself (a region in which the temperature rises as one goes to higher altitudes). Ozone thus plays a key role in the temperature structure of the Earth's atmosphere. Without the filtering action of the ozone layer, more of the Sun's UV-B radiation would penetrate the atmosphere and would reach the Earth's surface. Many experimental studies of plants and animals and clinical studies of humans have shown the harmful effects of excessive exposure to UV-B radiation.
At the Earth's surface, ozone comes into direct contact with life-forms and displays its destructive side. Because ozone reacts strongly with other molecules, high levels of ozone are toxic to living systems. Several studies have documented the harmful effects of ozone on crop production, forest growth, and human health. The substantial negative effects of surface-level tropospheric ozone from this direct toxicity contrast with the benefits of the additional filtering of UV-B radiation that it provides.
The dual role of ozone leads to two separate environmental issues. There is concern about increases in ozone in the troposphere. Low-lying ozone is a key component of photochemical smog, a familiar problem in the atmosphere of many cities around the world. Higher amounts of surface-level ozone are increasingly being observed in rural areas as well.
There is also widespread scientific and public interest and concern about losses of ozone in the stratosphere. Ground-based and satellite instruments have measured decreases in the amount of stratospheric ozone in our atmosphere. Over some parts of Antarctica, up to 60% of the total overhead amount of ozone (known as the column ozone) is depleted during Antarctic spring (September-November). This phenomenon is known as the Antarctic ozone hole. In the Arctic polar regions, similar processes occur that have also led to significant chemical depletion of the column ozone during late winter and spring in 6 out of the last 9 years. The ozone loss from January through late March has been typically 20-25%, and shorter-period losses have been higher, depending on the meteorological conditions encountered in the Arctic stratosphere. Smaller, but still significant, stratospheric ozone decreases have been seen at other, more-populated regions of the Earth. Increases in surface UV-B radiation have been observed in association with local decreases in stratospheric ozone, from both ground-based and satellite-borne instruments.
The scientific evidence, accumulated over more than two decades of study by the international research community, has shown that human-produced chemicals are responsible for the observed depletions of the ozone layer. The ozone-depleting compounds contain various combinations of the chemical elements chlorine, fluorine, bromine, carbon, and hydrogen and are often described by the general term halocarbons. The compounds that contain only chlorine, fluorine, and carbon are called chlorofluorocarbons, usually abbreviated as CFCs. CFCs, carbon tetrachloride, and methyl chloroform are important human-produced ozone-depleting gases that have been used in many applications including refrigeration, air conditioning, foam blowing, cleaning of electronics components, and as solvents. Another important group of human-produced halocarbons is the halons, which contain carbon, bromine, fluorine, and (in some cases) chlorine and have been mainly used as fire extinguishants. Governments have decided to eventually discontinue production of CFCs, halons, carbon tetrachloride, and methyl chloroform (except for a few special uses), and industry has developed more "ozone-friendly" substitutes.
Two responses are natural when a new problem has been identified: cure and prevention. When the problem is the destruction of the stratospheric ozone layer, the corresponding questions have been the following ones: Can we repair the damage already done? How can we prevent further destruction? Remedies have been investigated that could (1) remove CFCs selectively from the atmosphere, (2) intercept ozone-depleting chlorine before much depletion has taken place, or (3) replace the ozone lost in the stratosphere (perhaps by shipping the ozone from cities that have too much smog or by making new ozone). However, because ozone reacts strongly with other molecules, it is too unstable to be made elsewhere (e.g., in the smog of cities) and transported to the stratosphere. Considering the huge volume of the Earth's atmosphere and the magnitude of global stratospheric ozone depletion, the suggested remedies quickly become much too expensive, too energy consuming, impractical, and potentially damaging to the global environment.
Repair involves the internationally agreed-upon Montreal Protocol and its Amendments and Adjustments. This agreement regulates the production of CFCs and other ozone-depleting substances. Production of the most damaging ozone-depleting substances was eliminated, except for a few critical uses, by 1996 in developed countries and will be eliminated by 2010 in developing countries. As a result, the total concentration of chlorine in the lower atmosphere that can be carried to the stratosphere has peaked already. The concentrations in the stratosphere will likely peak by the end of this decade and then will start to decrease slowly as natural processes remove the ozone-depleting substances. All other things being equal, and with adherence to the international agreements, the ozone layer is expected to recover over the next 50 years or so.